α β γ Radiation
Therefore they have a mass of approximately where so the mass of an alpha particle is 6 64 10 27 kg 3 73 gev c 2.
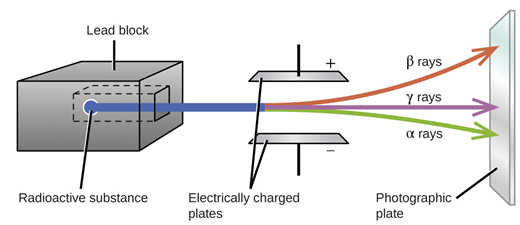
α β γ radiation. Other sources include x rays from medical radiography examinations and muons mesons positrons neutrons and other particles that constitute the secondary cosmic rays that are produced after primary cosmic rays interact with earth s atmosphere. With a worldwide growing number of nuclear power plants the next accident can be expected. A common source of ionizing radiation is radioactive materials that emit α β or γ radiation consisting of helium nuclei electrons or positrons and photons respectively. Have same rate of decay but they may have different effect on a medium when passing through it.
Hiroshima and nagasaki bikini atoll sellafield alexander litwinenko chernobyl fukushima radioactivity is lethal. This is associated. Beta radiation consists of fast moving electrons or positrons an antimatter electron. Mass of alpha beta and gamma radiation.
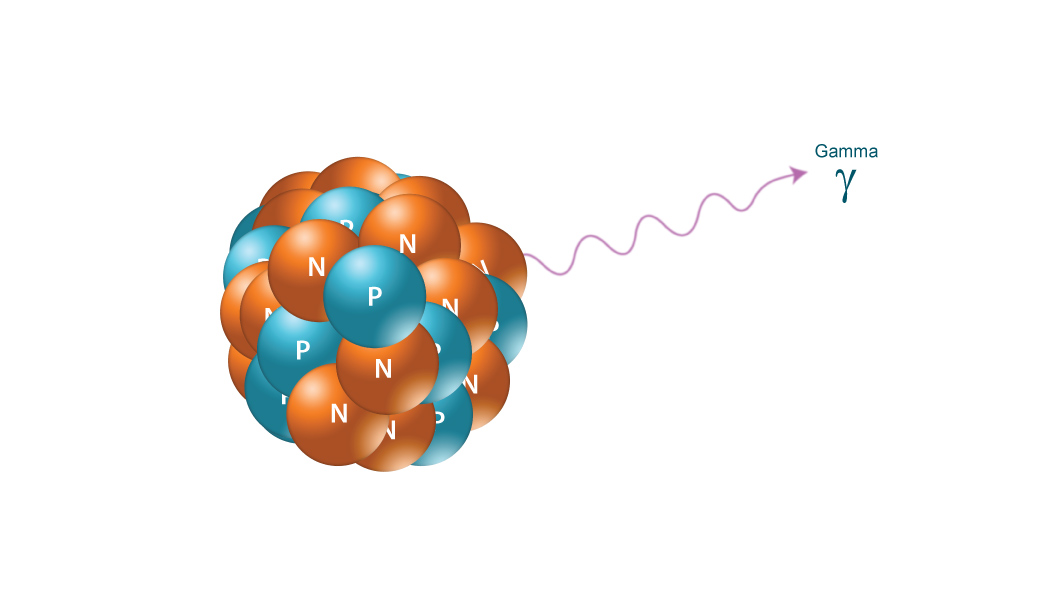
Only the interaction of α β and γ radiation with material will be discussed here. For a given energy alpha particles are much slower than beta particles giving rise to greater impulses. Alpha α radiation is the emission of 4 he nuclides while β radiation is the emission. All of them are considered to be forms of ionizing radiation that is when they strike atoms or molecules in their paths they cause electrons to be knocked away forming ions or free radicals.
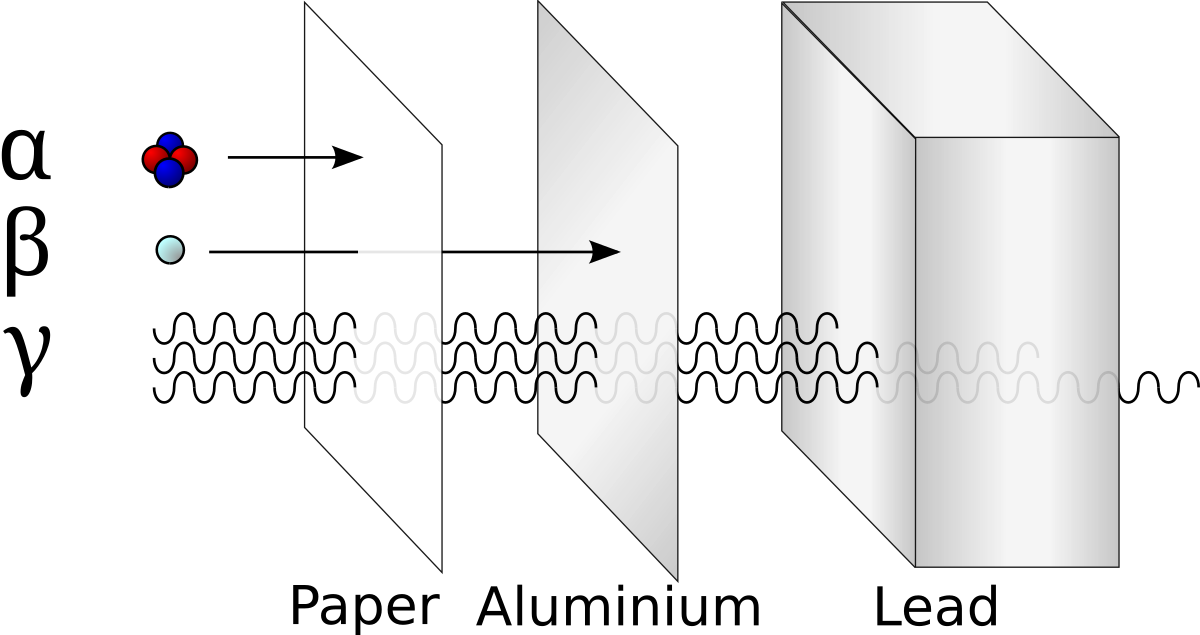
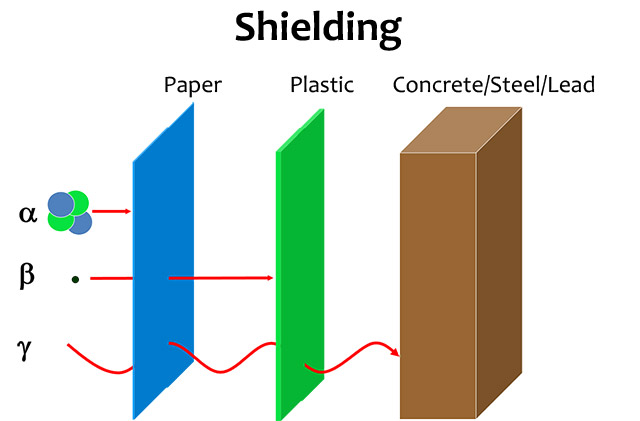
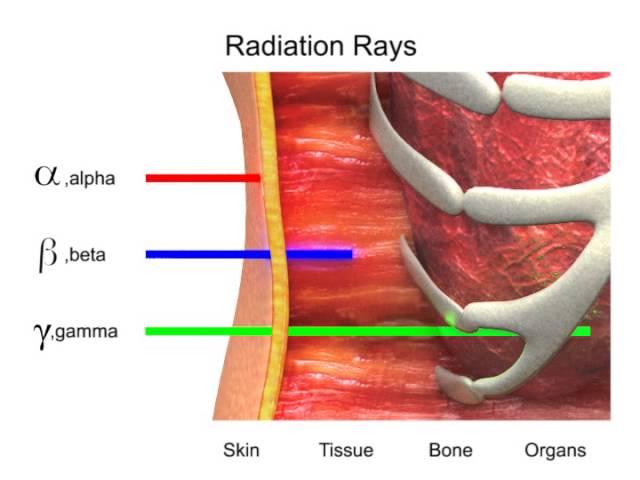
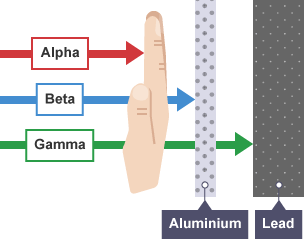
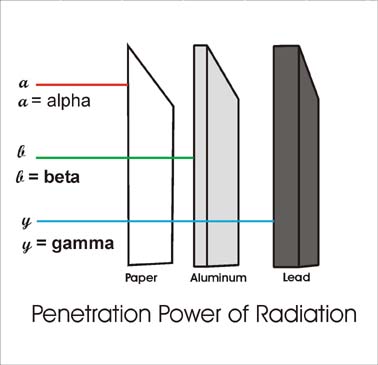
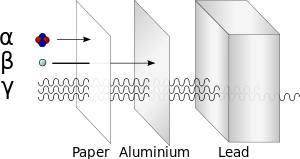
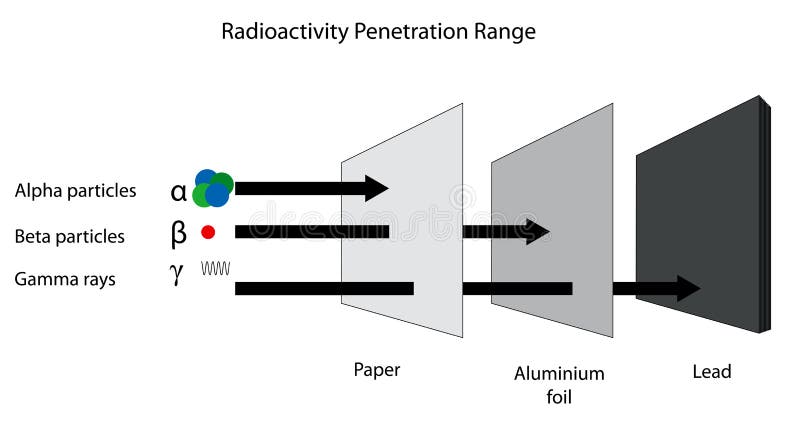
A beta particle is much lighter than an alpha and carries one unit of charge. Additionally its double charge 2e makes an alpha particle have a very high rate of energy loss in matter thus making it heavily ionizing radiation. Radiation α β γ or on its energy. For example alpha radiation travels only a few centimetres in air beta radiation travels tens of centimetres in air and gamma radiation travels very large distances.
Consequently the penetration depth of alpha particles is very small compared to the other radiations. Is known as as radiation absorbed dose rad. Based on the ability of radiation to ionize knock electrons from atom is known as roentgen r based on ionization in air. Alpha radiation consists of a stream of fast moving helium nuclei two protons and two neutrons.
Of the types of radiation produced in nuclear decay reactions the three most common are alpha particles α beta particles β and gamma rays γ. 1 ci of 137cs and 1 ci of 60co. Alpha particles are made of four nucleons. For low density materials the range.
Electrons and positrons which make up beta particles are antiparticles of each other. These three categories are labeled with the first three letters of the greek alphabet. The radiation of norm can also cause the formation of neutrons by the interaction of α particles with light elements which exist in building material. Pin photodiode α β γ radiation sensor.
However this effect can be neglected nearly in all scenarios. While this is generally not much of. Radioactivity is occasionally in the media. α alpha β beta and γ gamma.
Also often people where exposed without knowing for example while scavenging scrap metal.